Ice Cream Science
Scroll through the page to view examples of student work from the Ice Cream Science activity completed within SEE's Environmental Systems in the Outdoors Research Experience (ESORE). At the bottom of the page, there is a link for educators to access resources to try this activity with their students!
Claire Y.
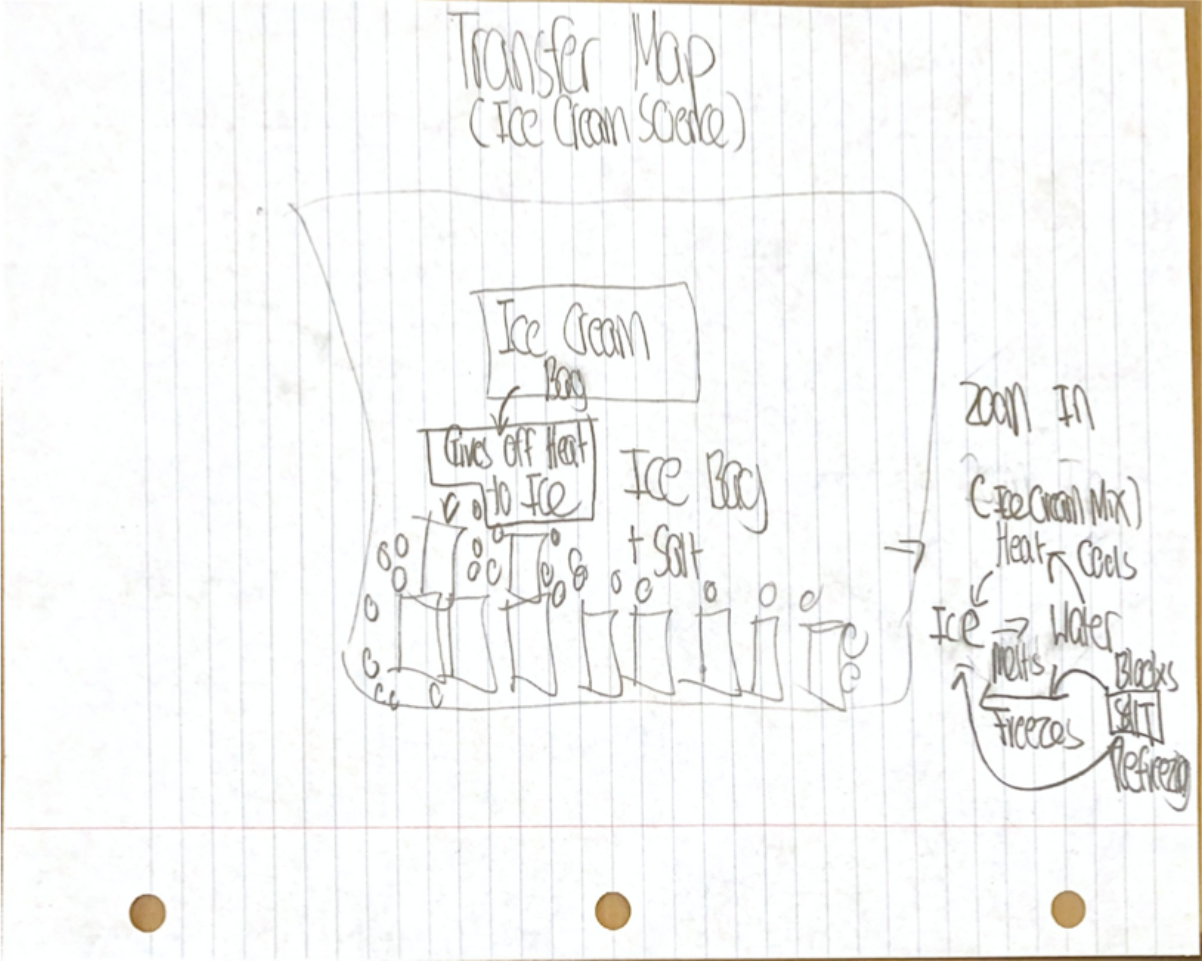
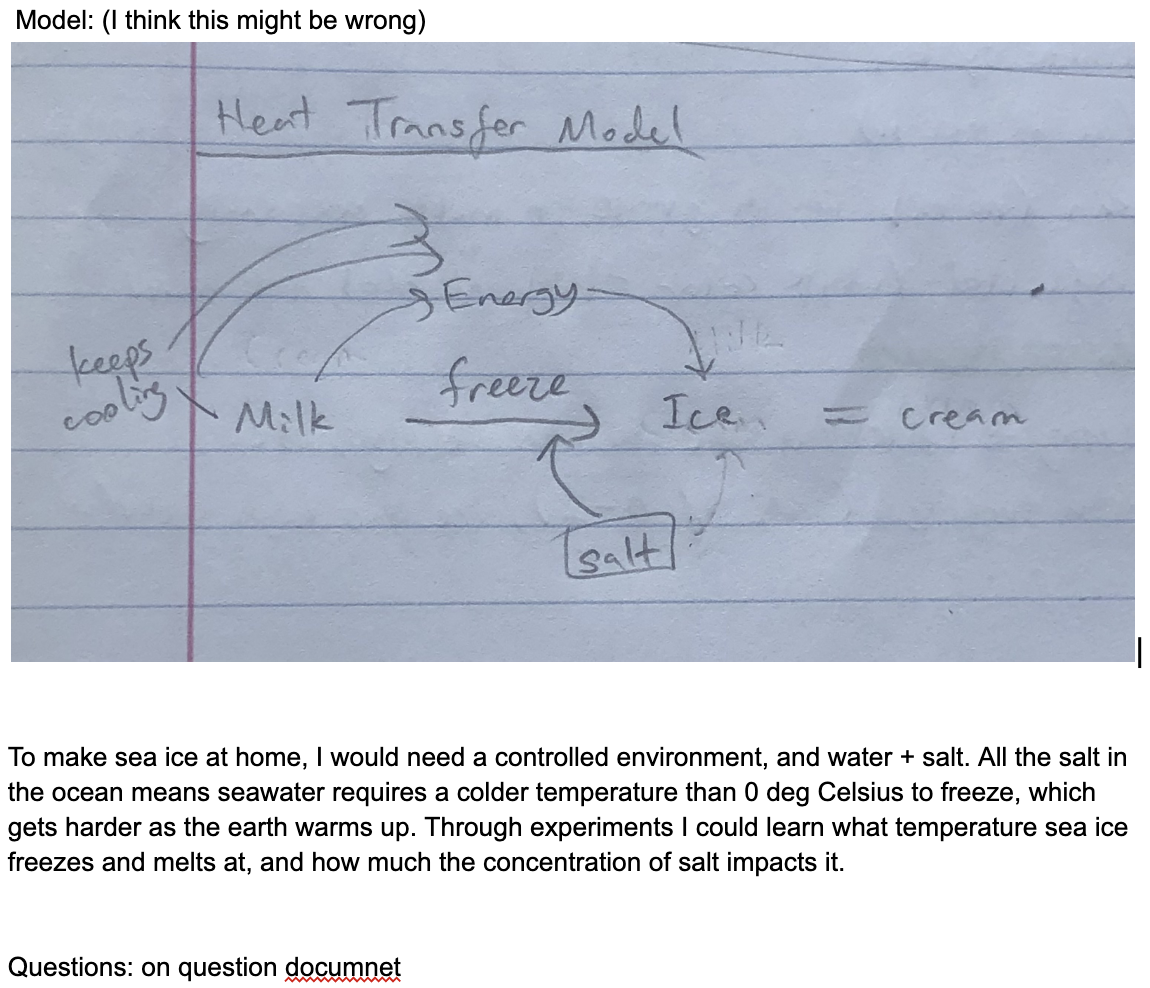
Model: (I think this might be wrong)
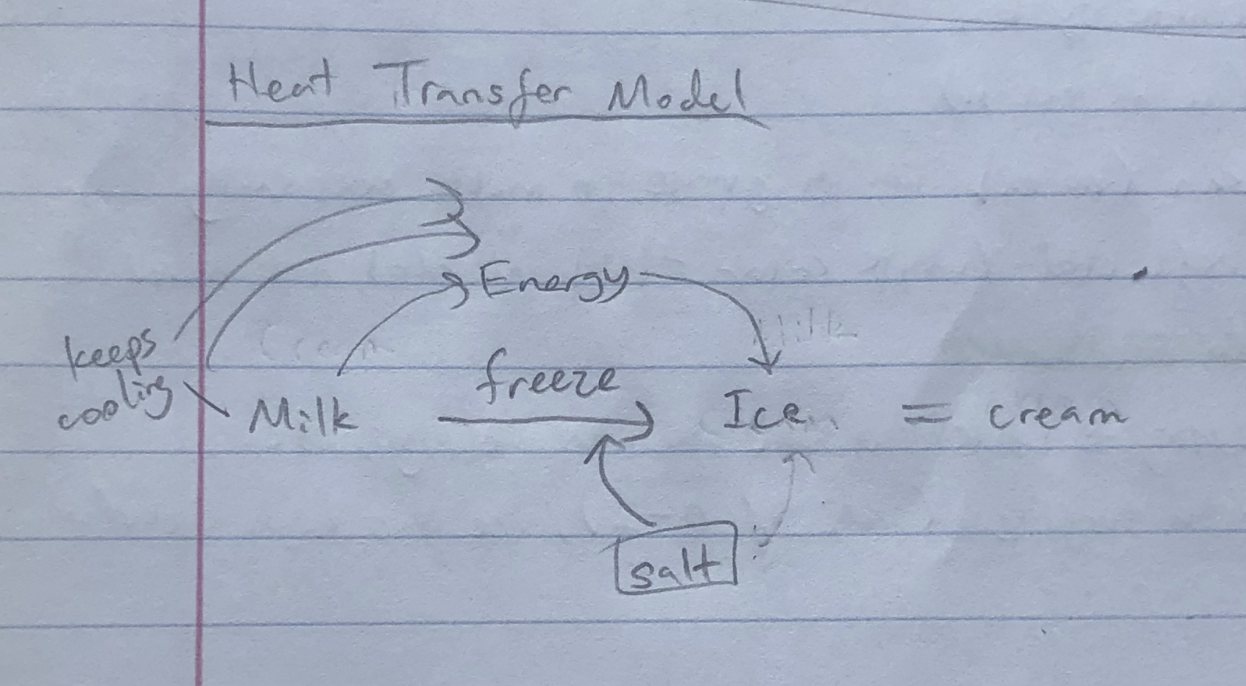
To make sea ice at home, I would need a controlled environment, and water + salt. All the salt in the ocean means seawater requires a colder temperature than 0 deg Celsius to freeze, which gets harder as the earth warms up. Through experiments I could learn what temperature sea ice freezes and melts at, and how much the concentration of salt impacts it.
Questions:
Would kelp farming be an effective and sustainable way to combat climate change in oceans?
Does the thickness and area of sea ice affect albedo or is it only based on color?
Is there any way to artificially alter the albedo of sea ice (somehow make its color lighter) to help it rrecover?
Old vs. new ice
SEA ICE @ HOME/Reproducible Sea Ice Sample: I would get a container and fill it up with tap water. Next, I would mix salt in with the tap water and then freeze the water in my freezer. I would like to experiment with the sea ice by:
- Introducing plastic/other materials into the ice (Effects of Pollution on Ice)
- Introducing various levels of Heat into the ice (Climate Change)
FOR EDUCATORS
Interested in learning more about this activity and trying it out with your students? Check out a blank copy of the worksheet for this activity HERE.